what is the result of applying too much of your sample to your tlc plate?
Thin Layer Chromatography (TLC)
TLC is a simple, quick, and inexpensive procedure that gives the chemist a quick answer as to how many components are in a mixture. TLC is as well used to support the identity of a compound in a mixture when the Rf of a chemical compound is compared with the Rf of a known compound (preferably both run on the aforementioned TLC plate).
A TLC plate is a canvass of glass, metal, or plastic which is coated with a thin layer of a solid adsorbent (usually silica or alumina). A small corporeality of the mixture to be analyzed is spotted about the bottom of this plate. The TLC plate is and then placed in a shallow puddle of a solvent in a developing sleeping room so that only the very bottom of the plate is in the liquid. This liquid, or the eluent, is the mobile phase, and it slowly rises up the TLC plate past capillary action.
As the solvent moves past the spot that was applied, an equilibrium is established for each component of the mixture between the molecules of that component which are adsorbed on the solid and the molecules which are in solution. In principle, the components will differ in solubility and in the strength of their adsorption to the adsorbent and some components will be carried further up the plate than others. When the solvent has reached the superlative of the plate, the plate is removed from the developing chamber, dried, and the separated components of the mixture are visualized. If the compounds are colored, visualization is straightforward. Usually the compounds are not colored, so a UV lamp is used to visualize the plates. (The plate itself contains a fluorescent dye which glows everywhere except where an organic compound is on the plate.)
How To Run a TLC Plate
 | Step ane: Prepare the developing containerThe developing container for TLC can be a specially designed bedroom, a jar with a lid, or a beaker with a sentinel drinking glass on the pinnacle (the latter is used in the undergrad labs at CU). Cascade solvent into the chamber to a depth of merely less than 0.5 cm. To help in the saturation of the TLC sleeping room with solvent vapors, you can line part of the inside of the beaker with filter paper. Cover the beaker with a spotter glass, swirl it gently, and permit it to stand while you prepare your TLC plate. |
 | Step two: Set the TLC plateTLC plates used in the organic chem educational activity labs are purchased as 5 cm x 20 cm sheets. Each large sheet is cutting horizontally into plates which are 5 cm tall by various widths; the more samples yous plan to run on a plate, the wider it needs to be. Shown in the photo to the left is a box of TLC plates, a large un-cut TLC sheet, and a pocket-sized TLC plate which has been cut to a user-friendly size. Handle the plates carefully then that yous do not disturb the coating of adsorbent or become them dirty. |
 | Measure 0.v cm from the bottom of the plate. Using a pencil, draw a line across the plate at the 0.five cm marker. This is the origin: the line on which you volition spot the plate. Take intendance not to press and so hard with the pencil that y'all disturb the adsorbent. Nether the line, mark lightly the name of the samples you lot will spot on the plate, or mark numbers for time points. Leave plenty space between the samples so that they do not run together; about iv samples on a 5 cm wide plate is advised. |
 | Footstep 3: Spot the TLC plateIf the sample is not already in solution, deliquesce about one mg in ane mL of a volatile solvent such equally hexanes, ethyl acetate, or methylene chloride. As a rule of thumb, a concentration of 1% ordinarily works well for TLC analysis. If the sample is also concentrated, it volition run every bit a smear or streak (come across troubleshooting section below); if it is not concentrated plenty, you volition encounter aught on the plate. Sometimes yous volition need to utilize trial and error to get well-sized, easy to read spots. |
 | Obtain a a microcapillary. In the organic teaching labs, we use 10µL microcaps - they are easier to handle than the smaller ones used in inquiry labs. Dip the microcap into the solution and and then gently touch the end of it onto the proper location on the TLC plate. Don't allow the spot to become too large - if necessary, you tin impact it to the plate, lift it off and blow on the spot. If you repeat these steps, the wet surface area on the plate will stay minor. |
 | This instance plate has been spotted with three different quantities of the same solution and is set to develop. If yous are unsure of how much sample to spot, you can ever spot multiple quantities and come across which looks all-time. |
 | Stride 4: Develop the platePlace the prepared TLC plate in the developing beaker, cover the beaker with the watch drinking glass, and leave it undisturbed on your bench top. The solvent will rise up the TLC plate by capillary action. Make sure the solvent does non cover the spot. |
 | Permit the plate to develop until the solvent is about half a centimeter beneath the peak of the plate. Remove the plate from the beaker and immediately mark the solvent front with a pencil. Allow the plate to dry. |
 | Step v: Visualize the spotsIf there are any colored spots, circumvolve them lightly with a pencil. Nearly samples are not colored and need to be visualized with a UV lamp. Hold a UV lamp over the plate and circle any spots you encounter. Beware! UV light is damaging both to your eyes and to your skin! Make certain you are wearing your goggles and exercise not look direct into the lamp. Protect your skin by wearing gloves. |
 | If the TLC plate runs samples which are too full-bodied, the spots will exist streaked and/or run together. If this happens, you will have to first over with a more dilute sample to spot and run on a TLC plate. |
 | Here's what overloaded plates look similar compared to well-spotted plates. The plate on the left has a big yellow smear; this smear contains the same two compounds which are nicely resolved on the plate next to information technology. |
TLC Solvents Choice
When y'all need to determine the best solvent or mixture of solvents (a "solvent organisation") to develop a TLC plate or chromatography column loaded with an unknown mixture, vary the polarity of the solvent in several trial runs: a process of trial and fault. Carefully detect and record the results of the chromatography in each solvent arrangement. You will find that as y'all increase the polarity of the solvent system, all the components of the mixture move faster (and vice versa with lowering the polarity). The platonic solvent system is simply the system that gives the all-time separation.
TLC elution patterns commonly carry over to column chromatography elution patterns. Since TLC is a much faster process than cavalcade chromatography, TLC is often used to make up one's mind the all-time solvent system for column chromatography. For instance, in determining the solvent system for a flash chromatography procedure, the ideal system is the one that moves the desired component of the mixture to a TLC Rf of 0.25-0.35 and will divide this component from its nearest neighbor past difference in TLC Rf values of at least 0.20. Therefore a mixture is analyzed by TLC to determine the ideal solvent(due south) for a flash chromatography procedure.
Beginners often practice not know where to offset: What solvents should they pull off the shelf to use to elute a TLC plate? Because of toxicity, toll, and flammability concerns, the common solvents are hexanes (or petroleum ethers/ligroin) and ethyl acetate (an ester). Diethyl ether can exist used, only it is very flammable and volatile. Alcohols (methanol, ethanol) can be used. Acetic acid (a carboxylic acid) can be used, usually as a small per centum component of the organization, since it is corrosive, non-volatile, very polar, and has irritating vapors. Acetone (a ketone) can exist used. Methylene chloride or and chloroform (halogenated hydrocarbons) are good solvents, only are toxic and should be avoided whenever possible. If two solvents are equal in performance and toxicity, the more volatile solvent is preferred in chromatography because it will exist easier to remove from the desired compound afterward isolation from a column chromatography procedure.
Ask the lab teacher what solvents are bachelor and advisable. Then, mix a not-polar solvent (hexanes, a mixture of 6-carbon alkanes) with a polar solvent (ethyl acetate or acetone) in varying percent combinations to make solvent systems of greater and lesser polarity. The charts below should assistance you lot in your solvent selection. You tin can also download this pdf chart of elution order. 

Interactions Between the Compound and the Adsorbent
The strength with which an organic compound binds to an adsorbent depends on the strength of the following types of interactions: ion-dipole, dipole-dipole, hydrogen bonding, dipole induced dipole, and van der Waals forces. With silica gel, the dominant interactive forces between the adsorbent and the materials to be separated are of the dipole-dipole type. Highly polar molecules collaborate fairly strongly with the polar SiOH groups at the surface of these adsorbents, and will tend to stick or adsorb onto the fine particles of the adsorbent while weakly polar molecules are held less tightly. Weakly polar molecules generally tend to move through the adsorbent more rapidly than the polar species. Roughly, the compounds follow the elution order given above.
The Rf value
The retention factor, or Rf, is divers equally the distance traveled past the chemical compound divided past the distance traveled by the solvent.

For instance, if a compound travels 2.1 cm and the solvent front end travels two.viii cm, the Rf is 0.75:
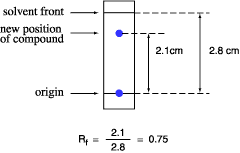
The Rf for a compound is a constant from one experiment to the side by side just if the chromatography weather below are besides abiding:
- solvent system
- adsorbent
- thickness of the adsorbent
- amount of material spotted
- temperature
Since these factors are difficult to keep constant from experiment to experiment, relative Rf values are generally considered. "Relative Rf" means that the values are reported relative to a standard, or it means that you compare the Rf values of compounds run on the same plate at the same time.
The larger an Rf of a compound, the larger the distance it travels on the TLC plate. When comparing two different compounds run nether identical chromatography conditions, the chemical compound with the larger Rf is less polar because it interacts less strongly with the polar adsorbent on the TLC plate. Conversely, if you lot know the structures of the compounds in a mixture, you can predict that a chemical compound of low polarity will take a larger Rf value than a polar compound run on the same plate.
The Rf tin provide corroborative evidence as to the identity of a compound. If the identity of a compound is suspected but not withal proven, an authentic sample of the chemical compound, or standard, is spotted and run on a TLC plate side by side (or on acme of each other) with the chemical compound in question. If two substances have the aforementioned Rf value, they are likely (but not necessarily) the same chemical compound. If they accept different Rf values, they are definitely different compounds. Note that this identity check must be performed on a single plate, considering it is difficult to duplicate all the factors which influence Rf exactly from experiment to experiment.
Troubleshooting TLC
All of the above (including the procedure folio) might sound similar TLC is quite an easy procedure. But what about the first time you run a TLC, and run into spots everywhere and blurred, streaked spots? As with whatever technique, with practice you go amend. Examples of common problems encountered in TLC:
- The compound runs equally a streak rather than a spot: The sample was overloaded. Run the TLC once again later on diluting your sample. Or, your sample might just contain many components, creating many spots which run together and appear as a streak. Perhaps, the experiment did not go as well as expected.
- The sample runs every bit a smear or a upwards crescent: Compounds which possess strongly acidic or basic groups (amines or carboxylic acids) sometimes show up on a TLC plate with this behavior. Add a few drops of ammonium hydroxide (amines) or acerb acid (carboxylic acids) to the eluting solvent to obtain clearer plates.
- The sample runs as a downward crescent: Probable, the adsorbent was disturbed during the spotting, causing the crescent shape.
- The plate solvent front runs crookedly: Either the adsorbent has flaked off the sides of the plate or the sides of the plate are touching the sides of the container (or the paper used to saturate the container) every bit the plate develops. Crooked plates brand it harder to measure Rf values accurately.
- Many random spots are seen on the plate: Make sure that yous exercise not accidentally drib any organic compound on the plate. If get a TLC plate and leave it laying on your workbench as y'all practice the experiment, you might driblet or splash an organic chemical compound on the plate.
- You see a mistiness of blue spots on the plate equally it develops: Perhaps you used an ink pen instead of a pencil to mark the origin?
- No spots are seen on the plate: Yous might not accept spotted enough compound, perhaps because the solution of the compound is too dilute. Endeavour concentrating the solution, or spot it several times in ane place, allowing the solvent to dry between applications. Some compounds practise non show up under UV light; try some other method of visualizing the plate (such equally staining or exposing to iodine vapor). Or, perhaps you do not take whatever compound because your experiment did not go equally well as planned. If the solvent level in the developing jar is deeper than the origin (spotting line) of the TLC plate, the solvent volition deliquesce the compounds into the solvent reservoir instead of allowing them to motion up the plate by capillary activity. Thus, you will not come across spots after the plate is developed. These photos testify how the yellowish compound is running into the solvent when lifted from the developing jar.

TLC Technique Quiz
Come across how well you understand TLC by taking the online TLC Technique Quiz!
Source: https://www.orgchemboulder.com/Technique/Procedures/TLC/TLC.shtml#:~:text=If%20the%20TLC%20plate%20runs,run%20on%20a%20TLC%20plate.
0 Response to "what is the result of applying too much of your sample to your tlc plate?"
ارسال یک نظر